Here, I will showcase work that I do(Information and pictures from the links below). :)
This was one of my work in my ACE project in term 2, where we were supposed to research on how a
solid’s properties will change near absolute zero.
Cryogenics (Study of solid’s properties near absolute zero)
Absolute zero is the
theoretical temperature at which the entropy reaches its bare minimum value.
The laws of thermodynamics states that absolute zero is impossible to be
achieved through merely thermodynamics means. Absolute zero is 0K and
-273.15˚C. Scientists have been able to attain temperatures very near the
absolute zero, causing matter to display quantum effects such as superfluidity
which is
a state of matter in which the matter behaves like a fluid with zero viscosity
(thickness) and zero entropy. The substance, which looks like a normal liquid,
will flow without resistance past any surface, which allows it to continue to move
over obstructions and through pores in containers which hold it, subject only
to its own inertia (force that makes an object stay in the same position/stop). Quantum effects like superconductivity which is a
phenomenon of exactly zero electrical resistance and expulsion of magnetic
fields occurring in certain materials when cooled under a characteristic
critical temperature would also occur. Bose-Einstein condensate is a state of
matter of a dilute gas of weakly interacting bosons confined in an external
potential and cooled to temperatures very close to absolute zero (0 K or
−273.15 °C[1]). Under such conditions, a large fraction of the
bosons occupy the lowest quantum state of the external potential, at which
point quantum effects become apparent on a macroscopic scale. These effects are
called macroscopic quantum phenomena.
Sources from: http://en.wikipedia.org/wiki/Absolute_zero
Reflections
I had prior knowledge on this topic because I read a Horrible Science book that taught me the laws of thermodynamics, the absolute zero et cetera, therefore, I chose to work on this because it is very interesting and I would like to find out more in depth about this topic.
I had prior knowledge on this topic because I read a Horrible Science book that taught me the laws of thermodynamics, the absolute zero et cetera, therefore, I chose to work on this because it is very interesting and I would like to find out more in depth about this topic.
This ACE project, I am supposed to make a visual display(i.e. posters, brochures, electronic posters) of examples of a solution, a heterogeneous solution, a colloid, and a suspension. After that, I am supposed to label each of them and explain their differences.
Brochure on solutions
(Homogeneous, heterogeneous, colloid and suspension)
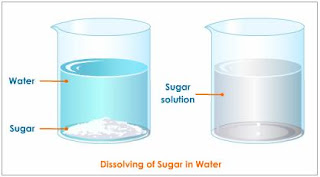
Homogeneous
·
Same
uniform appearance and composition throughout
·
Differs from heterogeneous mixture in size of particles
·
i.e. Sugar solution
Heterogeneous
·
Heterogeneous mixture consists of visibly
different substances or phases. The three phases or states of matter are gas,
liquid, and solid.
·
i.e. Air with clouds- clouds consist of tiny water
droplets
Colloids
· Homogeneous solution with intermediate particle
size between a solution and a suspension.
·
i.e. Milk, fog
Suspension
·
A
heterogeneous mixture of larger particles
·
i.e. Fine
sand, tomato juice
Reflections
This ACE was quite easy as there is only four relatively simple things to research on. I think this has aided me in the learning of Solutions and Suspensions topic as I knew beforehand their characteristics.
For this ACE project, I was also required to read a book and write a book review on it. It is under my Reading And Reasoning page. I received 4 marks for my work and I think all the hardwork paid off.
I also went for the ICAS Science Competition. The following is my results.
Reflections
I was quite disappointed for this competition because I was one mark away from Distinction. Now, I only received a credit. I hope that next time, I would be able to do better and perhaps aim 39/45! :)
This ACE was quite easy as there is only four relatively simple things to research on. I think this has aided me in the learning of Solutions and Suspensions topic as I knew beforehand their characteristics.
For this ACE project, I was also required to read a book and write a book review on it. It is under my Reading And Reasoning page. I received 4 marks for my work and I think all the hardwork paid off.
I also went for the ICAS Science Competition. The following is my results.
Reflections
I was quite disappointed for this competition because I was one mark away from Distinction. Now, I only received a credit. I hope that next time, I would be able to do better and perhaps aim 39/45! :)





No comments:
Post a Comment